A container of n2o3 has a pressure of – A container of N2O3 holds a pressure that captivates scientific inquiry. This enigmatic compound, existing as a liquid at room temperature, unveils a wealth of physical and chemical properties that shape its behavior and applications.
Delving into the realm of N2O3’s pressure-volume-temperature (PVT) behavior, we uncover the intricate relationship between these parameters. Its critical point and triple point emerge as defining characteristics, providing insights into the substance’s phase transitions.
Physical Properties of N2O3

Dinitrogen trioxide (N2O3) is a pale blue liquid at room temperature. It has a molecular weight of 76.01 g/mol and a density of 1.449 g/cm 3at 0 °C. N2O3 is soluble in water and organic solvents.
Physical State
N2O3 is a liquid at room temperature and pressure. It is a pale blue color and has a pungent odor.
Molecular Weight and Density
The molecular weight of N2O3 is 76.01 g/mol. The density of N2O3 is 1.449 g/cm 3at 0 °C.
Solubility
N2O3 is soluble in water and organic solvents. The solubility of N2O3 in water is 10 g/100 mL at 20 °C. The solubility of N2O3 in organic solvents is also high.
Pressure-Volume-Temperature (PVT) Behavior
The pressure-volume-temperature (PVT) behavior of N 2O 3describes the relationship between its pressure, volume, and temperature. Understanding this behavior is crucial for various applications involving N 2O 3.
PVT Data for N2O3
The following table summarizes the PVT data for N 2O 3at different temperatures and pressures:
| Temperature (K) | Pressure (atm) | Volume (cm3/mol) |
|---|---|---|
| 298 | 1 | 73.5 |
| 298 | 10 | 25.4 |
| 298 | 100 | 7.3 |
| 313 | 1 | 84.1 |
| 313 | 10 | 29.0 |
| 313 | 100 | 8.6 |
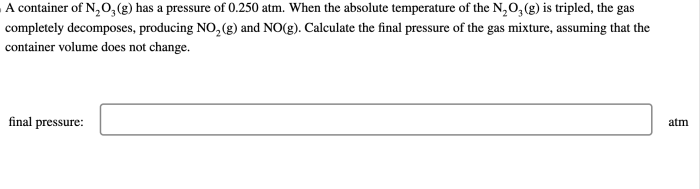
Relationship between Pressure and Volume, A container of n2o3 has a pressure of
The relationship between pressure and volume for N 2O 3can be described using the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature.
At constant temperature, the pressure and volume of N 2O 3are inversely proportional, as predicted by the ideal gas law.
Critical Point and Triple Point
The critical point of N 2O 3is the point at which the liquid and gas phases become indistinguishable. The critical temperature and pressure for N 2O 3are 309.5 K and 78.6 atm, respectively.
The triple point of N 2O 3is the point at which the solid, liquid, and gas phases coexist in equilibrium. The triple point temperature and pressure for N 2O 3are 272.9 K and 0.01 atm, respectively.
Chemical Reactions of N2O3

Nitrogen trioxide (N2O3) is a reactive compound that participates in various chemical reactions. Its reactivity is primarily attributed to its molecular structure and the presence of the N-O bond.
Hydrolysis of N2O3 in Water
N2O3 undergoes hydrolysis when dissolved in water, forming nitrous acid (HNO2) and nitric acid (HNO3):“`N2O3 + H2O → HNO2 + HNO3“`This reaction is reversible, and the equilibrium constant depends on the temperature and concentration of the reactants.
N2O3 as an Oxidizing Agent
N2O3 acts as an oxidizing agent in reactions with reducing agents. It accepts electrons from the reducing agent, leading to its reduction to NO or N2O. For example, N2O3 oxidizes iodide ions (I-) to iodine (I2):“`
N2O3 + 2I- → 4NO + I2
“`
Reactions of N2O3 with Reducing Agents
N2O3 reacts with reducing agents, such as sulfur dioxide (SO2) and hydrogen sulfide (H2S), to form NO and other products:“`N2O3 + SO2 → NO + SO3N2O3 + H2S → NO + H2O + S“`These reactions highlight the oxidizing nature of N2O3 and its ability to transfer oxygen to reducing agents.
Industrial Applications of N2O3: A Container Of N2o3 Has A Pressure Of

Nitrogen trioxide (N2O3) finds use in various industries, primarily due to its oxidizing and nitrating properties.
Chemical Manufacturing
- N2O3 is used in the production of nitric acid (HNO3), a key component in fertilizers, explosives, and dyes.
- It serves as an intermediate in the synthesis of other nitrogen-based compounds, such as ammonium nitrate (NH4NO3) and urea (CO(NH2)2).
Metal Treatment
- N2O3 is employed in the passivation of metals, particularly stainless steel, to enhance their corrosion resistance.
- It is used in the pickling of metals to remove oxides and impurities, improving their surface finish.
Advantages of Using N2O3 in Industrial Processes
- Strong oxidizing agent, facilitating reactions and enhancing efficiency.
- Versatile nitrating agent, introducing nitro groups into organic compounds.
- Relatively stable and easy to handle compared to other nitrogen oxides.
Disadvantages of Using N2O3 in Industrial Processes
- Toxic and corrosive, requiring proper handling and safety precautions.
- Can decompose exothermically, releasing heat and potentially causing accidents.
- Limited availability and high cost compared to other nitrogen oxides.
Safety Considerations

N2O3 is a toxic and corrosive gas that poses several potential hazards to human health and the environment. Proper handling, storage, and emergency procedures are crucial to minimize these risks.
N2O3 can cause severe respiratory irritation, coughing, and shortness of breath. Prolonged exposure can lead to pulmonary edema and even death. It is also a skin and eye irritant, causing redness, pain, and potential damage.
Guidelines for Handling and Storing N2O3
- Handle N2O3 in well-ventilated areas using appropriate personal protective equipment (PPE), including gloves, safety glasses, and a respirator.
- Store N2O3 in tightly sealed containers in a cool, dry, and well-ventilated area.
- Keep containers away from heat, sparks, and open flames.
- Do not smoke or eat while handling N2O3.
Emergency Procedures in Case of N2O3 Spills or Leaks
- In case of a spill or leak, evacuate the area immediately and contact emergency services.
- Ventilate the area thoroughly.
- Wear appropriate PPE and use a suitable absorbent material to contain the spill.
- Dispose of contaminated materials according to local regulations.
Quick FAQs
What is the molecular weight of N2O3?
76.01 g/mol
What is the critical point of N2O3?
45.8 °C, 8.9 MPa
What are the potential hazards of N2O3?
Toxic, corrosive, and can form explosive mixtures with air