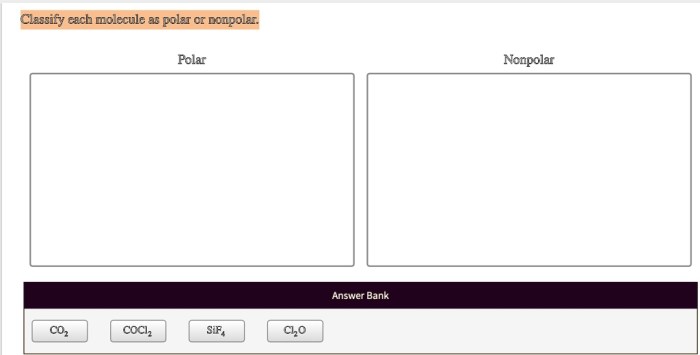
Classify each molecule as polar or nonpolar. – Polarity is a fundamental property of molecules that influences their behavior in various chemical and biological processes. This article presents a comprehensive guide to classifying molecules as polar or nonpolar, providing a clear understanding of the factors that determine molecular polarity and its wide-ranging applications.
The concept of molecular polarity stems from the uneven distribution of electrons within a molecule, resulting in the formation of a dipole. The molecular shape, electronegativity of constituent atoms, and bond lengths play crucial roles in determining the polarity of a molecule.
By understanding these factors, we can effectively classify molecules as polar or nonpolar, enabling us to predict their properties and interactions.
Molecular Structure and Polarity
Molecular polarity refers to the uneven distribution of electrons within a molecule, creating a separation of positive and negative charges. This polarity is determined by the electronegativity of the atoms involved and the molecular geometry.
The electronegativity of an atom is its ability to attract electrons towards itself. When atoms with different electronegativities bond, the more electronegative atom will pull the shared electrons closer to itself, creating a polar bond. The polarity of a molecule is the vector sum of the polarities of its individual bonds.
Relationship between Molecular Shape and Polarity
The shape of a molecule also influences its polarity. A symmetrical molecule, such as carbon dioxide (CO2), has a zero net dipole moment because the polarities of the individual bonds cancel each other out. In contrast, an asymmetrical molecule, such as water (H2O), has a non-zero net dipole moment due to the bent shape of the molecule.
Classifying Molecules as Polar or Nonpolar: Classify Each Molecule As Polar Or Nonpolar.
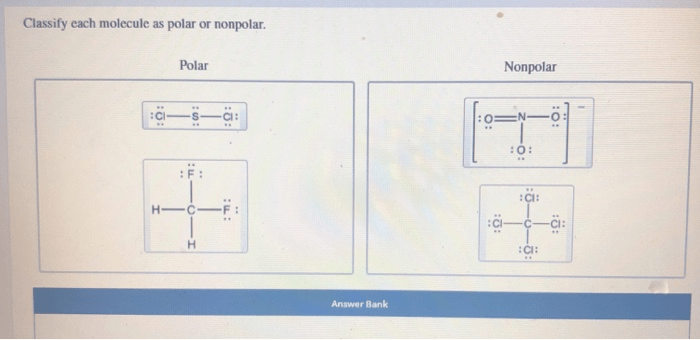
To classify a molecule as polar or nonpolar, we can use the following method:
- Determine the electronegativity difference between the bonded atoms.
- If the difference is greater than 0.5, the bond is considered polar.
- If the difference is less than or equal to 0.5, the bond is considered nonpolar.
The following table provides examples of polar and nonpolar molecules along with their corresponding structures:
| Molecule | Structure | Polarity |
|---|---|---|
| Water (H2O) |  |
Polar |
| Carbon dioxide (CO2) |  |
Nonpolar |
| Hydrogen chloride (HCl) |  |
Polar |
| Methane (CH4) |  |
Nonpolar |
Factors Influencing Polarity
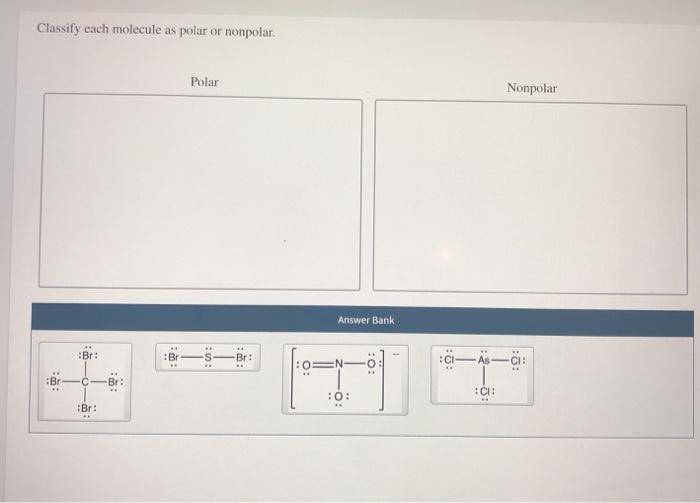
The polarity of a molecule is influenced by the following factors:
Electronegativity
As mentioned earlier, the electronegativity difference between bonded atoms determines the polarity of a bond. The greater the electronegativity difference, the more polar the bond.
Bond Length
The bond length also affects polarity. A shorter bond length indicates a stronger bond, which means that the electrons are held more tightly by the more electronegative atom. This results in a more polar bond.
Applications of Molecular Polarity
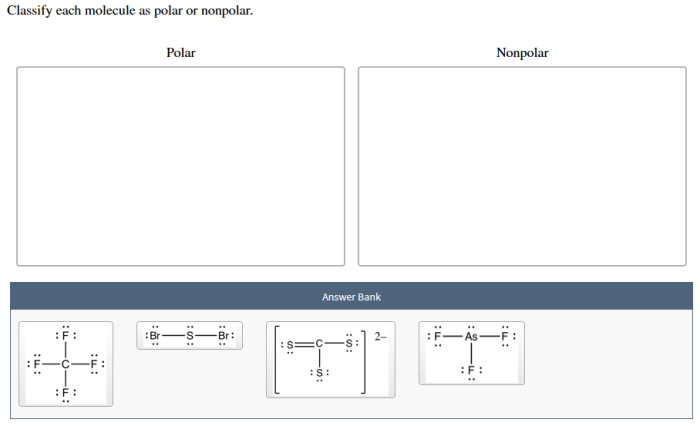
Molecular polarity has numerous applications in various fields, including:
Chemistry
- Predicting the solubility of substances
- Designing new materials with specific properties
- Understanding reaction mechanisms
Biology, Classify each molecule as polar or nonpolar.
- Determining the structure and function of proteins
- Understanding the interactions between cells
- Developing new drugs and therapies
Materials Science
- Designing new materials with tailored properties
- Understanding the behavior of materials under different conditions
- Developing new technologies for energy storage and conversion
Polarity in Intermolecular Interactions
Molecular polarity plays a crucial role in intermolecular interactions, such as:
Dipole-Dipole Interactions
Dipole-dipole interactions occur between polar molecules that have a permanent dipole moment. These interactions are attractive in nature and contribute to the cohesive forces between molecules.
Hydrogen Bonding
Hydrogen bonding is a particularly strong type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom, such as oxygen or nitrogen. Hydrogen bonds are responsible for the unique properties of water and many other substances.
Effect on Physical Properties
Polarity influences the physical properties of substances, such as:
- Boiling point: Polar substances have higher boiling points than nonpolar substances due to the stronger intermolecular forces.
- Solubility: Polar substances are generally more soluble in polar solvents than in nonpolar solvents.
Top FAQs
What is the significance of molecular polarity?
Molecular polarity governs the intermolecular interactions between molecules, influencing their physical properties such as boiling point, solubility, and reactivity.
How does molecular shape affect polarity?
Molecular shape determines the spatial arrangement of electronegative and electropositive regions within a molecule, influencing the overall polarity.
What are the applications of understanding molecular polarity?
Understanding molecular polarity enables the design of materials with specific properties, facilitates drug development, and aids in understanding biological processes.